
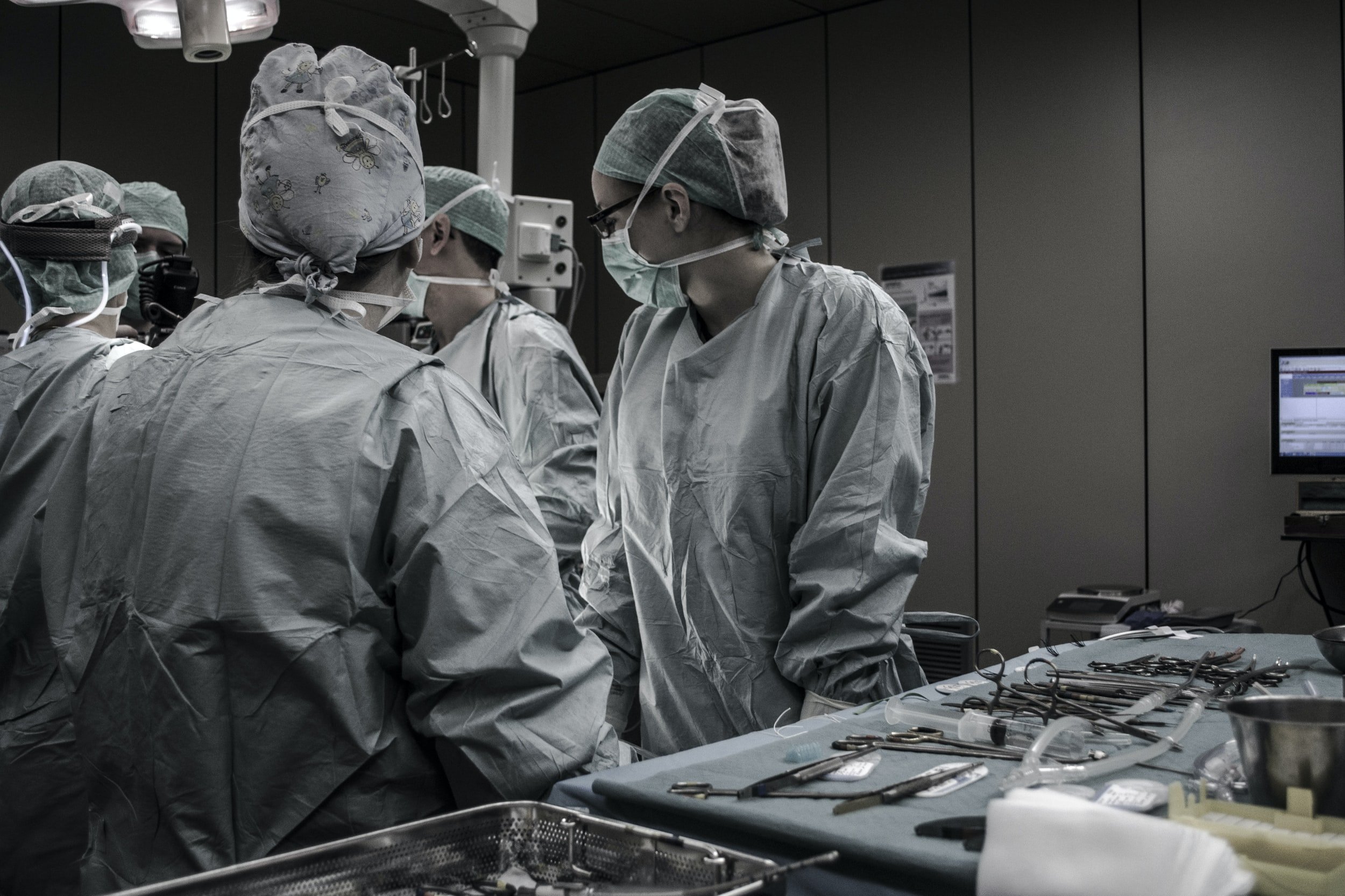
For Surgeons Who Demand Greater Control
Simple and Minimally Invasive Surgical Technology to Address Hammertoes

What is TenoTac® 2.0?
TenoTac® is a patent-pending procedure and soft tissue fixation system designed to permanently address the source of the toe contracture. Cannulated instrumentation allows for accurate and reproducible placement of the implant. A threaded titanium implant with a spiked base is used to achieve and hold soft tissue fixation. The procedure requires minimal steps to correct flexible and semi-rigid hammertoe, plantar plate insufficiency, MPJ instability, as well as other applications in the forefoot. Instrumentation facilitates a two-handed technique to achieve correction, temporary fixation, and final implant placement. The implant features a low-profile dorsal component to reduce soft tissue irritation which threads into a plantar tack with a single row of spikes intended to grip and hold tension on the flexor tendon. The system is available in sterile kit offers two dorsal lengths to accommodate varying patient anatomies.

“It is an indispensable procedure for any foot and ankle surgeon and the Paragon 28® TenoTac® has more than refined this century old procedure.”
- Douglas Blacklidge, DPM
A Simple &
Reproducible Technique
TenoTac® 2.0 Enhancements:
Paragon 28 launched the TenoTac® 2.0 system to provide for greater capture of soft tissue, streamlined tensioning , and improved fit of the implant to the bony surface.
Plantar component includes a washer to allow it to angulate up to 20 degrees to contour to the plantar phalanx to maximize the amount of surface area the implant has on the flexor tendons and bone
Plantar component diameter increased to ensure increased capture of all four bands of the flexor tendons
Plantar component tooth height increased to allow for better capture and increased hold of the flexor tendons
Plantar inserter includes a sleeve to flush the drill tunnel of debris
Dorsal sleeve and plantar tack are tapered to allow for an easier mating of the components
Dorsal component is rectangular in shape with an increased surface area to limit plunging
Dorsal component has teeth to allow for capture of the dorsal cortex to limit plunging and to allow for the dorsal component to bite into the cortex while tightening to limit prominence
Methods of Fixation
The TenoTac® 2.0 Soft Tissue Fixation System is a unique offering due to its versatility and options for fixation. Depending on the specific digital deformity, the flexor digitorum brevis (FDB) or flexor digitorum longus (FDL) can be included or isolated to achieve desired correction. In addition, surgeons have the opportunity to increase or decrease tension applied to specific tendons to fine-tune correction if needed.
Explore some impressive results from this minimally invasive solution for hammertoe, claw toe, mallet toe, crossover toe, and floating toe.
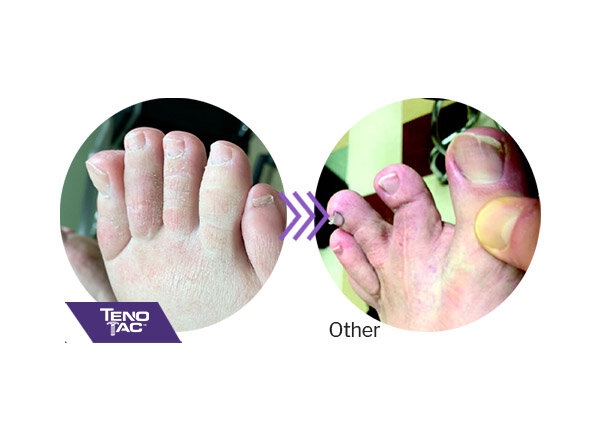
TenoTac maintains soft tissue attachments, lowering the chance of hyper-extension of the toe
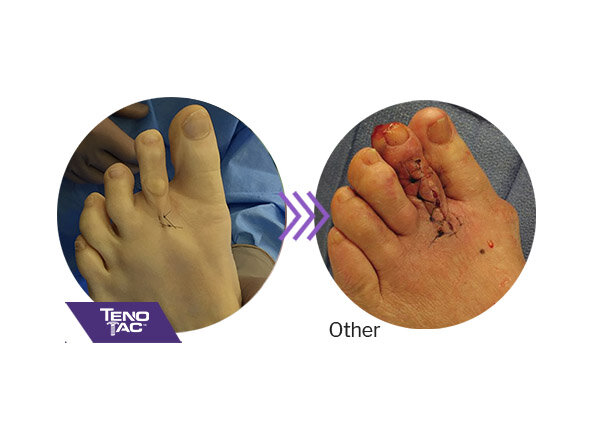
TenoTac is less invasive than traditional tendon transfer procedures and may result in less post-operative swelling
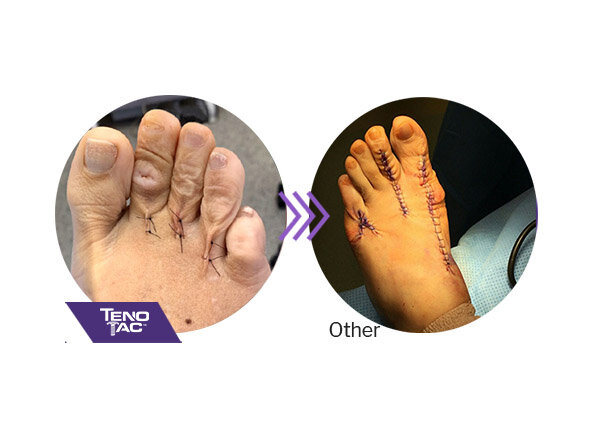
TenoTac requires a shorter dorsal and plantar incision than other procedures and may result in less postoperative scarring
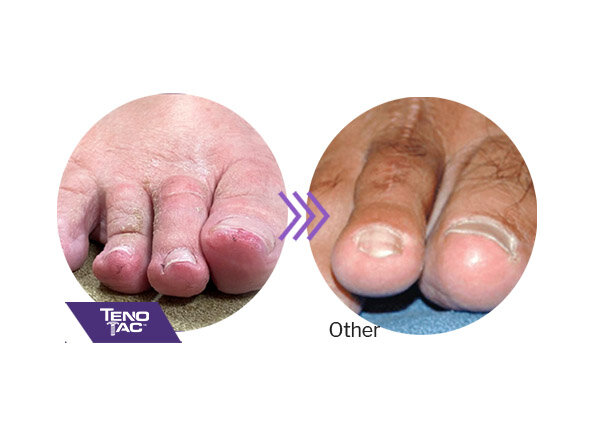
TenoTac balances the toe, minimizing the chance of floating toe
Implant Features
Cannulated instrumentation
Threaded titanium implant with a spiked base to achieve soft tissue fixation
Minimal steps to correct flexible and semi-rigid hammertoes, MTP instability, supplementation for plantar plate insufficiency and rigid hammertoe for arthrodesis, as well as other applications in the foot and ankle
Instrumentation facilitates a two-handed technique to achieve correction, temporary fixation, and final implant placement
System designed to use a single, reproducible technique to correct a multitude of patient-specific pathologies
System Instrumentation
Ø0.9 mm K-wire
Ø2.8 mm Cannulated Drill/Standard Countersink with Handle
Dorsal Sleeve Driver
Plantar Tack Inserter
Dorsal Sleeve Standard Length Gauge
Kit Configuration
TenoTac® 2.0 Soft Tissue Fixation System Caddy
The TenoTac® 2.0 System is available in a sterile kit configuration which contains all implant sizes and necessary instrumentation.
Find A Rep Near You.
Interested in learning more about TenoTac® 2.0? Email us directly and we will connect you with the closest TenoTac representative in your area.
Apply to Be On Our Surgeon Finder for Patients
We are now offering the unique opportunity to be on our Surgeon Finder for our patient-facing website for TenoTac® 2.0. Please select one of the options below to get started.
If you are a part of our surgeon finder currently and would like to be removed, please click here and contact us.

















